HIV and protease inhibitors: Are there new opportunities at second line and beyond?

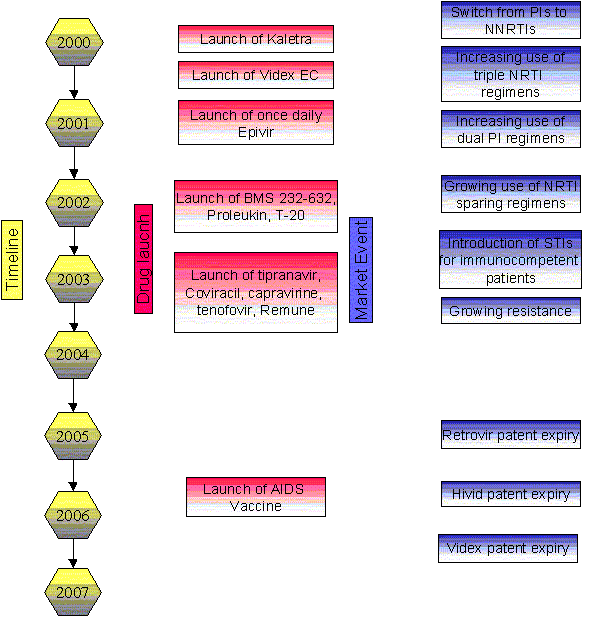
The tide of physician opinion turned against the protease inhibitors (PI) in 1999, which only a few years previously were heralded as the great saviors of HIV therapy. The catalyst of this change in treatment patterns was the launch of new products of the non-nucleoside reverse transcriptase inhibitor (NNRTI) class and the nucleoside reverse transcriptase inhibitor (NRTI) class, which have dosing, side-effect and price advantages over the PIs.
However, recent research with HIV physicians across the 7 major pharmaceutical markets carried out by Datamonitor at the end of 2000 reveals that switching away from the PIs is slowing down. The launch of Abbott's Kaletra (lopinavir/ritonavir) and the growing use of so-called "boosted PI" regimens are encouraging physicians to take a second look at this class and Datamonitor's research suggests that the doctors like what they see.
Datamonitor's new HIV market opportunity forecasting models, EpiCast HIV, reveal:
- Sales of the NRTIs Ziagen and Trizivir and the NNRTI Sustiva are growing at the expense of the PIs
- NNRTIs and triple NRTI regimens will remain the gold standard first line treatment, but opportunities for PIs to be used at later lines of therapy are growing
Sales of the recently launched NRTIs Ziagen and Trizivir and the NNRTI Sustiva have grown at the expense of the PIs
In 2000, DuPont's Sustiva (efavirenz) generated $386m worth of sales, a growth of 86% on its sales in 1999.
Natasha Jenkins, Datamonitor Infectious Disease Analyst, comments: "Sustiva's rapid uptake has been driven by the drug's advantages over both other NNRTIs and the PIs".
In terms of in-class competition, Sustiva has benefited from aggressive promotion by DuPont based on the drug's superior efficacy in the provision of sustained viral suppression in comparison to its competitors Viramune (nevirapine) and Rescriptor (delaviridine) and its superior dosing schedule.
The greatest driver of Sustiva's growth has undoubtedly been the product's advantages over the protease inhibitors. Sustiva has been marketed by DuPont as a PI-sparing drug, allowing physicians to postpone the use of potent regimens containing PIs until late stage HIV. The advantage of this treatment strategy is that in early stages of treatment, patients can benefit from the superior dosing, side effect profile and lower price of Sustiva, while receiving treatment which is as efficacious as the PIs in the early stages of HIV. When Sustiva becomes less efficacious, as a result of resistance, patients can be switched to a PI which is potent in late stage HIV.
As if the threat of Sustiva wasn't enough for the PIs, another recent entrant into the HIV market, Ziagen (abacavir), has created the opportunity for physicians to use another kind of PI-sparing regimen, one containing three NRTIs. In 2000, Ziagen achieved sales of $154m. Uptake of this product has been driven by the dosing advantages of a triple NRTI regimen over one containing two NRTIs and a PI. The recent launch of Glaxo Wellcome's (now GlaxoSmithKline) Trizivir (abacavir, zidovudine, lamivudine), which combines Ziagen and the company's other NRTIs, Epvir (lamivudine) and Retrovir (zidovudine) in one pill, will further increase the convenience of this treatment option and could potentially further damage sales of the PIs.
NRTIs and triple NRTI regimens will remain the gold standard first line treatment, but opportunities for PIs to be used at later lines of therapy are growing
The position of the NNRTIs and triple NRTIs regimens as first line treatments of choice are unlikely to be eroded. However, new developments in dosing or PIs means that they are becoming an increasingly attractive treatment choice for patients beyond first line therapy, which is opening up a whole new market opportunity for this class, particularly as the size of the late stage treatment market is the most rapidly growing in HIV. The fact that cross-resistance between NNRTIs limits their use to once in a patient's lifetime is another fact contributing to this ‘second look' at the PI class.
One of the most important events impacting the PI segment of the HIV market over the period 2000-2007 will be the growing use of regimens containing dual, rather than single PIs. The combination of PIs with low doses of ritonavir raises plasma levels of the main PI in a patient's blood. This increases the efficacy of the PI and allows for a more convenient dosing regimens, thereby enhancing an existing advantage of this class (its potency) and reducing one of its disadvantages (the inconvenience of dosing schedules). The adoption of this new treatment strategy will lead to a decline in the use of regimens containing Crixivan (indinavir), Agenerase (amprenavir), Fortovase (saquinavir) and Invirase (saquinavir) on their own and growing use of these drugs in combination with low doses of ritonavir.
In particular Abbott's recently launched Kaletra, which integrates its main ingredient, lopinavir, and ritonavir in one pill will benefit from the growing trend towards dual PI use in this market. The trend towards growing use of dual PI regimens is likely to be viewed by the companies involved as beneficial, as it improves the suitability of their products for use in later stage patients, rather than for use as first or second line therapies, for which they were originally positioned.
Natasha Jenkins, Datamonitor Infectious Disease Analyst, comments: "In the face of growing use of NNRTIs and NRTIs at first line, the repositioning of the PIs has allowed companies to reverse a trend which could potentially have seriously damaged their market share."
For further information on this press release, please contact Elisabeth Overend-Freeman at efreeman@datamonitor.com .
EpiCast HIV is an interactive forecasting tool for the HIV market, providing a flexible, interactive model of the commercial opportunities in HIV/AIDS treatment in the 7 leading pharmaceutical markets to 2007, in terms of epidemiology, patient share forecasts of combinations and individual drugs and drug sales forecasts.
Combining in-depth research with prescribing physicians and opinion leaders with in-house HIV market modeling expertise, EpiCast HIV allows exclusive insight into the drivers behind the HIV market, supporting forecasts with analysis and commercial recommendations. For further information or a demonstration of the models, please contact njenkins@datamonitor.com .
Datamonitor plc is an independent market analysis firm that publishes a wide portfolio of strategic business information. Datamonitor has expertise in the following industry sectors: Automotive & Transport; Consumer; Financial Services; Healthcare; Industrial; Medical Devices; Technology. Datamonitor can be contacted at (212) 686-7400 or visit www.datamonitor.com .
Source: Datamonitor
Subscribe to our free e-mail newsletter.
Click for a free Buyer's Guide listing.
